Class information for: |
Basic class information |
| Class id | #P | Avg. number of references |
Database coverage of references |
|---|---|---|---|
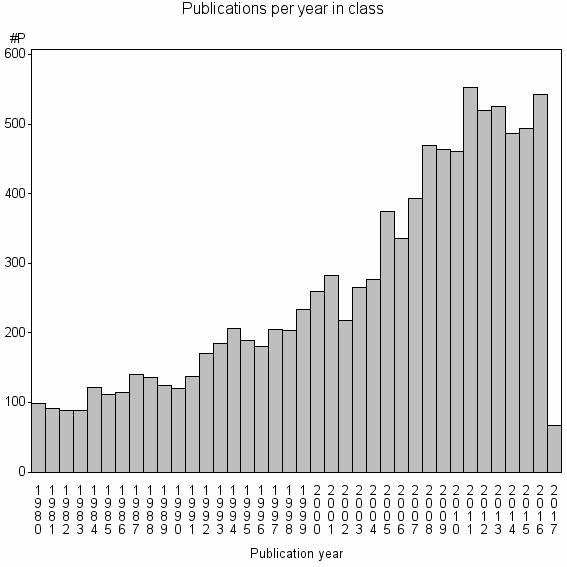
| 1075 | 9928 | 22.7 | 55% |
Hierarchy of classes |
The table includes all classes above and classes immediately below the current class. |
Terms with highest relevance score |
| rank | Term | termType | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|---|
| 1 | ADAPTIVE DESIGN | authKW | 696505 | 3% | 66% | 342 |
| 2 | STATISTICS IN MEDICINE | journal | 680488 | 13% | 18% | 1259 |
| 3 | STATISTICS & PROBABILITY | WoSSC | 565614 | 58% | 3% | 5744 |
| 4 | JOURNAL OF BIOPHARMACEUTICAL STATISTICS | journal | 445138 | 4% | 40% | 364 |
| 5 | MULTIPLE TESTING | authKW | 432187 | 3% | 52% | 271 |
| 6 | FALSE DISCOVERY RATE | authKW | 400000 | 3% | 47% | 280 |
| 7 | FAMILYWISE ERROR RATE | authKW | 397604 | 2% | 74% | 174 |
| 8 | INTERIM ANALYSIS | authKW | 363064 | 2% | 78% | 151 |
| 9 | SAMPLE SIZE | authKW | 330173 | 5% | 24% | 452 |
| 10 | PHARMACEUTICAL STATISTICS | journal | 314866 | 2% | 44% | 232 |
Web of Science journal categories |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | Statistics & Probability | 565614 | 58% | 3% | 5744 |
| 2 | Mathematical & Computational Biology | 228223 | 28% | 3% | 2767 |
| 3 | Medical Informatics | 154173 | 15% | 3% | 1533 |
| 4 | Medicine, Research & Experimental | 19413 | 17% | 0% | 1715 |
| 5 | Public, Environmental & Occupational Health | 14103 | 16% | 0% | 1627 |
| 6 | Pharmacology & Pharmacy | 7233 | 18% | 0% | 1767 |
| 7 | Health Care Sciences & Services | 6118 | 6% | 0% | 567 |
| 8 | Biology | 3485 | 6% | 0% | 591 |
| 9 | Psychology, Mathematical | 3106 | 1% | 1% | 133 |
| 10 | Computer Science, Interdisciplinary Applications | 962 | 3% | 0% | 328 |
Address terms |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | BIOMETR 1 | 116076 | 0% | 79% | 48 |
| 2 | MED PHARMACEUT STAT UNIT | 84186 | 1% | 46% | 59 |
| 3 | STAT | 56424 | 11% | 2% | 1112 |
| 4 | OFF BIOSTAT | 54815 | 1% | 14% | 132 |
| 5 | BIOSTAT | 40902 | 11% | 1% | 1084 |
| 6 | MED BIOMETRY UNIT | 31612 | 0% | 86% | 12 |
| 7 | OTS CDER | 30737 | 0% | 100% | 10 |
| 8 | BIOMETR 4 | 28818 | 0% | 45% | 21 |
| 9 | BIOSTAT PROGRAMMING | 24409 | 0% | 31% | 26 |
| 10 | CDER | 23873 | 1% | 14% | 54 |
Journals |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | STATISTICS IN MEDICINE | 680488 | 13% | 18% | 1259 |
| 2 | JOURNAL OF BIOPHARMACEUTICAL STATISTICS | 445138 | 4% | 40% | 364 |
| 3 | PHARMACEUTICAL STATISTICS | 314866 | 2% | 44% | 232 |
| 4 | BIOMETRICAL JOURNAL | 194927 | 4% | 16% | 388 |
| 5 | CONTROLLED CLINICAL TRIALS | 180645 | 2% | 25% | 239 |
| 6 | STATISTICS IN BIOPHARMACEUTICAL RESEARCH | 167556 | 1% | 42% | 129 |
| 7 | BIOMETRICS | 109204 | 4% | 9% | 401 |
| 8 | CLINICAL TRIALS | 105797 | 2% | 22% | 160 |
| 9 | DRUG INFORMATION JOURNAL | 84539 | 2% | 16% | 174 |
| 10 | STATISTICAL METHODS IN MEDICAL RESEARCH | 52126 | 1% | 15% | 112 |
Author Key Words |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | ADAPTIVE DESIGN | 696505 | 3% | 66% | 342 | Search ADAPTIVE+DESIGN | Search ADAPTIVE+DESIGN |
| 2 | MULTIPLE TESTING | 432187 | 3% | 52% | 271 | Search MULTIPLE+TESTING | Search MULTIPLE+TESTING |
| 3 | FALSE DISCOVERY RATE | 400000 | 3% | 47% | 280 | Search FALSE+DISCOVERY+RATE | Search FALSE+DISCOVERY+RATE |
| 4 | FAMILYWISE ERROR RATE | 397604 | 2% | 74% | 174 | Search FAMILYWISE+ERROR+RATE | Search FAMILYWISE+ERROR+RATE |
| 5 | INTERIM ANALYSIS | 363064 | 2% | 78% | 151 | Search INTERIM+ANALYSIS | Search INTERIM+ANALYSIS |
| 6 | SAMPLE SIZE | 330173 | 5% | 24% | 452 | Search SAMPLE+SIZE | Search SAMPLE+SIZE |
| 7 | MULTIPLE COMPARISONS | 264966 | 2% | 43% | 199 | Search MULTIPLE+COMPARISONS | Search MULTIPLE+COMPARISONS |
| 8 | GROUP SEQUENTIAL DESIGN | 264856 | 1% | 96% | 90 | Search GROUP+SEQUENTIAL+DESIGN | Search GROUP+SEQUENTIAL+DESIGN |
| 9 | SAMPLE SIZE RE ESTIMATION | 264473 | 1% | 98% | 88 | Search SAMPLE+SIZE+RE+ESTIMATION | Search SAMPLE+SIZE+RE+ESTIMATION |
| 10 | CONDITIONAL POWER | 254038 | 1% | 92% | 90 | Search CONDITIONAL+POWER | Search CONDITIONAL+POWER |
Core articles |
The table includes core articles in the class. The following variables is taken into account for the relevance score of an article in a cluster c: (1) Number of references referring to publications in the class. (2) Share of total number of active references referring to publications in the class. (3) Age of the article. New articles get higher score than old articles. (4) Citation rate, normalized to year. |
| Rank | Reference | # ref. in cl. |
Shr. of ref. in cl. |
Citations |
|---|---|---|---|---|
| 1 | BAUER, P , BRETZ, F , DRAGALIN, V , KONIG, F , WASSMER, G , (2016) TWENTY-FIVE YEARS OF CONFIRMATORY ADAPTIVE DESIGNS: OPPORTUNITIES AND PITFALLS.STATISTICS IN MEDICINE. VOL. 35. ISSUE 3. P. 325 -347 | 124 | 94% | 7 |
| 2 | ANTONIOU, M , JORGENSEN, AL , KOLAMUNNAGE-DONA, R , (2016) BIOMARKER-GUIDED ADAPTIVE TRIAL DESIGNS IN PHASE II AND PHASE III: A METHODOLOGICAL REVIEW.PLOS ONE. VOL. 11. ISSUE 2. P. - | 99 | 86% | 2 |
| 3 | RUTTERFORD, C , COPAS, A , ELDRIDGE, S , (2015) METHODS FOR SAMPLE SIZE DETERMINATION IN CLUSTER RANDOMIZED TRIALS.INTERNATIONAL JOURNAL OF EPIDEMIOLOGY. VOL. 44. ISSUE 3. P. 1051 -1067 | 81 | 84% | 2 |
| 4 | ONDRA, T , DMITRIENKO, A , FRIEDE, T , GRAF, A , MILLER, F , STALLARD, N , POSCH, M , (2016) METHODS FOR IDENTIFICATION AND CONFIRMATION OF TARGETED SUBGROUPS IN CLINICAL TRIALS: A SYSTEMATIC REVIEW.JOURNAL OF BIOPHARMACEUTICAL STATISTICS. VOL. 26. ISSUE 1. P. 99 -119 | 65 | 90% | 2 |
| 5 | VANDEMEULEBROECKE, M , (2008) GROUP SEQUENTIAL AND ADAPTIVE DESIGNS - A REVIEW OF BASIC CONCEPTS AND POINTS OF DISCUSSION.BIOMETRICAL JOURNAL. VOL. 50. ISSUE 4. P. 541 -557 | 76 | 100% | 12 |
| 6 | ROSENBERGER, WF , SVERDLOV, O , HU, FF , (2012) ADAPTIVE RANDOMIZATION FOR CLINICAL TRIALS.JOURNAL OF BIOPHARMACEUTICAL STATISTICS. VOL. 22. ISSUE 4. P. 719 -736 | 69 | 96% | 10 |
| 7 | TANNIOU, J , VAN DER TWEEL, I , TEERENSTRA, S , ROES, KCB , (2016) SUBGROUP ANALYSES IN CONFIRMATORY CLINICAL TRIALS: TIME TO BE SPECIFIC ABOUT THEIR PURPOSES.BMC MEDICAL RESEARCH METHODOLOGY. VOL. 16. ISSUE . P. - | 65 | 97% | 0 |
| 8 | GOEMAN, JJ , SOLARI, A , (2014) MULTIPLE HYPOTHESIS TESTING IN GENOMICS.STATISTICS IN MEDICINE. VOL. 33. ISSUE 11. P. 1946-1978 | 59 | 81% | 22 |
| 9 | BRETZ, F , KOENIG, F , BRANNATH, W , GLIMM, E , POSCH, M , (2009) ADAPTIVE DESIGNS FOR CONFIRMATORY CLINICAL TRIALS.STATISTICS IN MEDICINE. VOL. 28. ISSUE 8. P. 1181 -1217 | 54 | 96% | 60 |
| 10 | ALOSH, M , BRETZ, F , HUQUE, M , (2014) ADVANCED MULTIPLICITY ADJUSTMENT METHODS IN CLINICAL TRIALS.STATISTICS IN MEDICINE. VOL. 33. ISSUE 4. P. 693-713 | 50 | 100% | 7 |
Classes with closest relation at Level 2 |