Biomarker Development
High-throughput technological platforms are currently used to explore blood composition in microliter amount of serum or plasma and identify proteins as disease biomarkers.
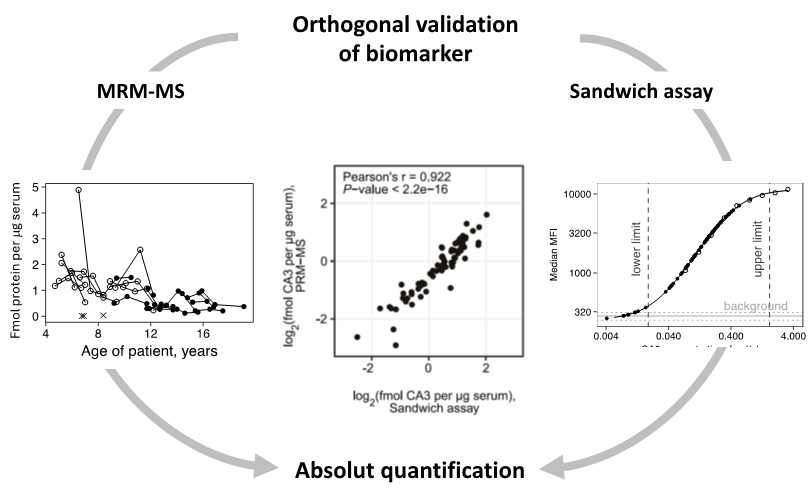
Patients affected by monogenic diseases would benefit from reliable and easily accessed blood biomarkers.Neuromuscular dystrophies like Duchenne muscular dystrophy requires a life-long health care. Currently, both clinical management of the disease and drug development rely on timed physical tests. Our team focuses on finding specific and informative biomarkers and developing sensitive and accurate assays to quantify them. Using biotechnological tools we aim to improve health care of patients.
Publications
-
Passarelli C, et al. 2020 Tumor Necrosis Factor Receptor SF10A (TNFRSF10A) SNPs Correlate With Corticosteroid Response in Duchenne Muscular Dystrophy. Front Genet 11:605.
-
Signorelli M, et al. 2019 Longitudinal serum biomarker screening identifies malate dehydrogenase 2 as candidate prognostic biomarker for Duchenne muscular dystrophy J Cachexia, Sarcopenia and Muscle DOI: 10.1002/jcsm.12517
-
Spitali P, et al. 2018 Tracking disease progression non‐invasively in Duchenne and Becker muscular dystrophies. J. Cachexia, Sarcopenia and Muscle. 9:715–726, DOI:10.1002/jcsm.12304
-
A. Lourbakos, et al. 2017 Evaluation of serum MMP-9 as predictive biomarker for antisense therapy in Duchenne, Sci Rep. 2017; 7: 17888, doi: 10.1038/s41598-017-17982-y.
-
Ayoglu B, et al. 2014 Profiling geographically dispersed cohorts reveals protein markers in blood for Duchenne muscular dystrophies. (2014) EMBO Mol. Med. 6:918-936.
