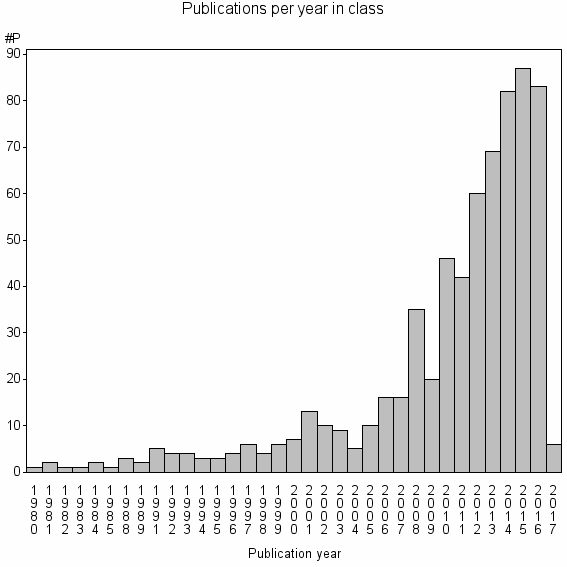
Class information for: |
Basic class information |
| Class id | #P | Avg. number of references |
Database coverage of references |
|---|---|---|---|
| 16184 | 668 | 27.9 | 50% |
Hierarchy of classes |
The table includes all classes above and classes immediately below the current class. |
| Cluster id | Level | Cluster label | #P |
|---|---|---|---|
| 1 | 4 | ECONOMICS//EDUCATION & EDUCATIONAL RESEARCH//PSYCHOL | 3876184 |
| 320 | 3 | AMERICAN JOURNAL OF PHARMACEUTICAL EDUCATION//MEDICATION ERRORS//AMERICAN JOURNAL OF HOSPITAL PHARMACY | 39312 |
| 1464 | 2 | FOOD AND DRUG LAW JOURNAL//CONFLICT OF INTEREST//ORPHAN DRUGS | 7747 |
| 16184 | 1 | ORPHAN DRUGS//RARE DISEASES//ORPHAN DRUG ACT | 668 |
Terms with highest relevance score |
| rank | Term | termType | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|---|
| 1 | ORPHAN DRUGS | authKW | 2942854 | 17% | 56% | 116 |
| 2 | RARE DISEASES | authKW | 1758582 | 25% | 23% | 164 |
| 3 | ORPHAN DRUG ACT | authKW | 454408 | 2% | 76% | 13 |
| 4 | OFF ORPHAN PROD DEV | address | 386243 | 2% | 65% | 13 |
| 5 | ORPHAN MEDICINAL PRODUCTS | authKW | 279974 | 1% | 88% | 7 |
| 6 | ORPHAN DESIGNATION | authKW | 182841 | 1% | 100% | 4 |
| 7 | ADAPTIVE LICENSING | authKW | 163248 | 1% | 71% | 5 |
| 8 | CONDITIONAL APPROVAL | authKW | 163248 | 1% | 71% | 5 |
| 9 | EUROPEAN REFERENCE NETWORKS | authKW | 163248 | 1% | 71% | 5 |
| 10 | ORPHANET JOURNAL OF RARE DISEASES | journal | 138421 | 9% | 5% | 58 |
Web of Science journal categories |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | Health Care Sciences & Services | 2699 | 14% | 0% | 93 |
| 2 | Health Policy & Services | 2240 | 10% | 0% | 65 |
| 3 | Pharmacology & Pharmacy | 2134 | 34% | 0% | 227 |
| 4 | Medicine, Research & Experimental | 740 | 13% | 0% | 89 |
| 5 | Genetics & Heredity | 462 | 12% | 0% | 81 |
| 6 | Medical Informatics | 359 | 3% | 0% | 20 |
| 7 | Medical Ethics | 306 | 1% | 0% | 9 |
| 8 | Medicine, General & Internal | 299 | 11% | 0% | 75 |
| 9 | Law | 289 | 4% | 0% | 28 |
| 10 | Public, Environmental & Occupational Health | 210 | 9% | 0% | 57 |
Address terms |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | OFF ORPHAN PROD DEV | 386243 | 2% | 65% | 13 |
| 2 | COORDINATING PIEDMONT AOSTA VALLEY NETWORK | 91421 | 0% | 100% | 2 |
| 3 | EUROPEAN GAUCHER ALLIANCE | 91421 | 0% | 100% | 2 |
| 4 | SHIRE HUMAN GENET THER IES | 91421 | 0% | 100% | 2 |
| 5 | SPAINRDR | 91421 | 0% | 100% | 2 |
| 6 | PEDIAT NEUROL METAB MED | 66483 | 1% | 36% | 4 |
| 7 | ORDR | 60946 | 0% | 67% | 2 |
| 8 | OFF RARE DIS | 60930 | 1% | 13% | 10 |
| 9 | ADV MANAGEMENT TRAINING PROGRAMME PHARMACOECON PH | 45710 | 0% | 100% | 1 |
| 10 | ALTALANOS ORVOSTUDOMANYI KAR GENOMIKAI MED RITK | 45710 | 0% | 100% | 1 |
Journals |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | ORPHANET JOURNAL OF RARE DISEASES | 138421 | 9% | 5% | 58 |
| 2 | EXPERT OPINION ON ORPHAN DRUGS | 17572 | 2% | 3% | 13 |
| 3 | FOOD AND DRUG LAW JOURNAL | 16597 | 3% | 2% | 19 |
| 4 | THERAPEUTIC INNOVATION & REGULATORY SCIENCE | 15215 | 2% | 3% | 11 |
| 5 | EJHP PRACTICE | 11242 | 1% | 4% | 7 |
| 6 | DRUG INFORMATION JOURNAL | 9346 | 2% | 1% | 15 |
| 7 | CLINICAL PHARMACOLOGY & THERAPEUTICS | 9043 | 5% | 1% | 34 |
| 8 | PUBLIC HEALTH GENOMICS | 8770 | 1% | 2% | 8 |
| 9 | DRUG DISCOVERY TODAY | 4168 | 2% | 1% | 15 |
| 10 | EXPERT REVIEW OF PHARMACOECONOMICS & OUTCOMES RESEARCH | 3227 | 1% | 1% | 6 |
Author Key Words |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | ORPHAN DRUGS | 2942854 | 17% | 56% | 116 | Search ORPHAN+DRUGS | Search ORPHAN+DRUGS |
| 2 | RARE DISEASES | 1758582 | 25% | 23% | 164 | Search RARE+DISEASES | Search RARE+DISEASES |
| 3 | ORPHAN DRUG ACT | 454408 | 2% | 76% | 13 | Search ORPHAN+DRUG+ACT | Search ORPHAN+DRUG+ACT |
| 4 | ORPHAN MEDICINAL PRODUCTS | 279974 | 1% | 88% | 7 | Search ORPHAN+MEDICINAL+PRODUCTS | Search ORPHAN+MEDICINAL+PRODUCTS |
| 5 | ORPHAN DESIGNATION | 182841 | 1% | 100% | 4 | Search ORPHAN+DESIGNATION | Search ORPHAN+DESIGNATION |
| 6 | ADAPTIVE LICENSING | 163248 | 1% | 71% | 5 | Search ADAPTIVE+LICENSING | Search ADAPTIVE+LICENSING |
| 7 | CONDITIONAL APPROVAL | 163248 | 1% | 71% | 5 | Search CONDITIONAL+APPROVAL | Search CONDITIONAL+APPROVAL |
| 8 | EUROPEAN REFERENCE NETWORKS | 163248 | 1% | 71% | 5 | Search EUROPEAN+REFERENCE+NETWORKS | Search EUROPEAN+REFERENCE+NETWORKS |
| 9 | ORPHAN DISEASES | 130587 | 1% | 29% | 10 | Search ORPHAN+DISEASES | Search ORPHAN+DISEASES |
| 10 | NEW DRUG LAUNCHES | 102847 | 0% | 75% | 3 | Search NEW+DRUG+LAUNCHES | Search NEW+DRUG+LAUNCHES |
Core articles |
The table includes core articles in the class. The following variables is taken into account for the relevance score of an article in a cluster c: (1) Number of references referring to publications in the class. (2) Share of total number of active references referring to publications in the class. (3) Age of the article. New articles get higher score than old articles. (4) Citation rate, normalized to year. |
| Rank | Reference | # ref. in cl. |
Shr. of ref. in cl. |
Citations |
|---|---|---|---|---|
| 1 | ZELEI, T , MOLNAR, MJ , SZEGEDI, M , KALO, Z , (2016) SYSTEMATIC REVIEW ON THE EVALUATION CRITERIA OF ORPHAN MEDICINES IN CENTRAL AND EASTERN EUROPEAN COUNTRIES.ORPHANET JOURNAL OF RARE DISEASES. VOL. 11. ISSUE . P. - | 45 | 85% | 0 |
| 2 | GAMMIE, T , LU, CY , BABAR, ZUD , (2015) ACCESS TO ORPHAN DRUGS: A COMPREHENSIVE REVIEW OF LEGISLATIONS, REGULATIONS AND POLICIES IN 35 COUNTRIES.PLOS ONE. VOL. 10. ISSUE 10. P. - | 28 | 97% | 6 |
| 3 | GUTIERREZ, L , PATRIS, J , HUTCHINGS, A , COWELL, W , (2015) PRINCIPLES FOR CONSISTENT VALUE ASSESSMENT AND SUSTAINABLE FUNDING OF ORPHAN DRUGS IN EUROPE.ORPHANET JOURNAL OF RARE DISEASES. VOL. 10. ISSUE . P. - | 24 | 89% | 2 |
| 4 | HERDER, M , (2013) WHEN EVERYONE IS AN ORPHAN: AGAINST ADOPTING A US-STYLED ORPHAN DRUG POLICY IN CANADA.ACCOUNTABILITY IN RESEARCH-POLICIES AND QUALITY ASSURANCE. VOL. 20. ISSUE 4. P. 227 -269 | 30 | 56% | 6 |
| 5 | HOEKMAN, J , BOON, WPC , BOUVY, JC , EBBERS, HC , DE JONG, JP , DE BRUIN, ML , (2015) USE OF THE CONDITIONAL MARKETING AUTHORIZATION PATHWAY FOR ONCOLOGY MEDICINES IN EUROPE.CLINICAL PHARMACOLOGY & THERAPEUTICS. VOL. 98. ISSUE 5. P. 534 -541 | 17 | 89% | 4 |
| 6 | MARTINALBO, J , BOWEN, D , CAMARERO, J , CHAPELIN, M , DEMOLIS, P , FOGGI, P , JONSSON, B , LLINARES, J , MOREAU, A , O'CONNOR, D , ET AL (2016) EARLY MARKET ACCESS OF CANCER DRUGS IN THE EU.ANNALS OF ONCOLOGY. VOL. 27. ISSUE 1. P. 96 -105 | 18 | 62% | 6 |
| 7 | PAULDEN, M , STAFINSKI, T , MENON, D , MCCABE, C , (2015) VALUE-BASED REIMBURSEMENT DECISIONS FOR ORPHAN DRUGS: A SCOPING REVIEW AND DECISION FRAMEWORK.PHARMACOECONOMICS. VOL. 33. ISSUE 3. P. 255 -269 | 22 | 69% | 3 |
| 8 | RODRIGUEZ-MONGUIO, R , SPARGO, T , SEOANE-VAZQUEZ, E , (2017) ETHICAL IMPERATIVES OF TIMELY ACCESS TO ORPHAN DRUGS: IS POSSIBLE TO RECONCILE ECONOMIC INCENTIVES AND PATIENTS' HEALTH NEEDS?.ORPHANET JOURNAL OF RARE DISEASES. VOL. 12. ISSUE . P. - | 21 | 72% | 0 |
| 9 | PINTO, D , MARTIN, D , CHENHALL, R , (2016) THE INVOLVEMENT OF PATIENT ORGANISATIONS IN RARE DISEASE RESEARCH: A MIXED METHODS STUDY IN AUSTRALIA.ORPHANET JOURNAL OF RARE DISEASES. VOL. 11. ISSUE . P. - | 22 | 52% | 4 |
| 10 | LOGVISS, K , KRIEVINS, D , PURVINA, S , (2016) IMPACT OF ORPHAN DRUGS ON LATVIAN BUDGET.ORPHANET JOURNAL OF RARE DISEASES. VOL. 11. ISSUE . P. - | 14 | 93% | 0 |
Classes with closest relation at Level 1 |