The P21.2 branch
The P21.2 branch was designed specifically for materials engineering and surface processes
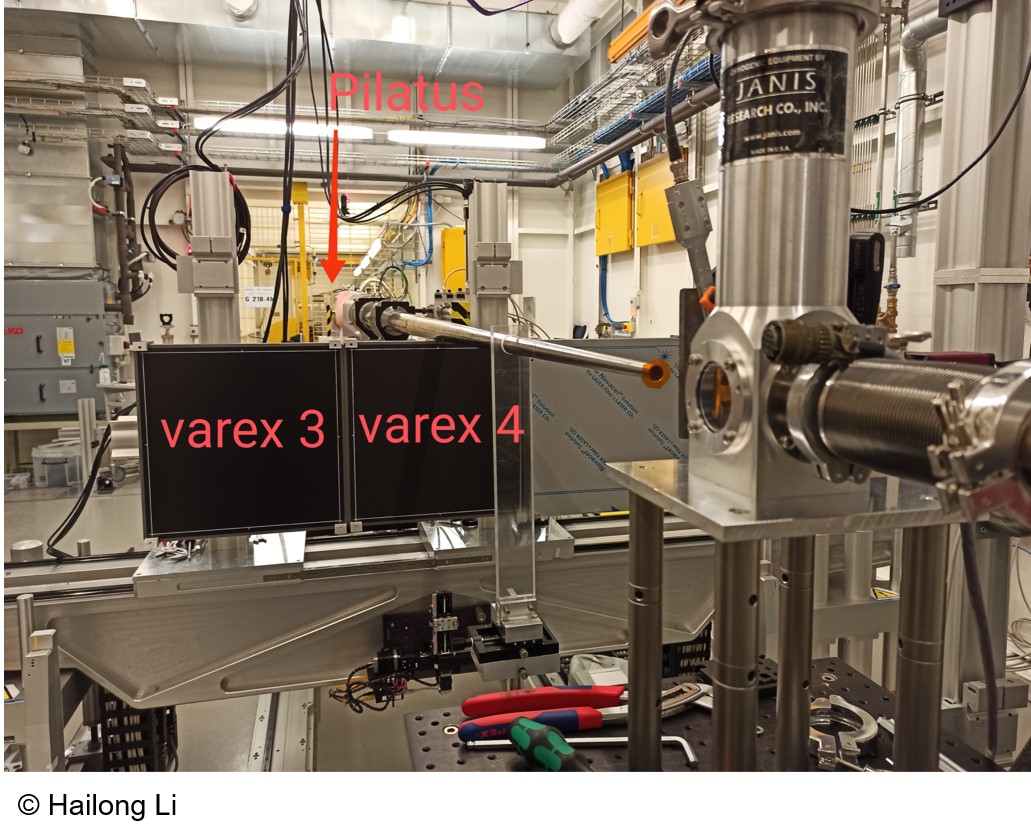
The Swedish Material Science beamline's P21.2 branch has two experimental hutches. Experimental Hutch 2 is currently being developed in this Roll-in station project.
Experimental Hutch ID Card
The ID Card of the P21.2 branch's experimental hutch 3 is:
- Energy range 38-150 keV
Simultaneous SAXS and WAXS (or WAXS or SAXS)
- Imaging
- “Zoom-in” data acquisition
- Beam size 2 by 6 mm ->1 by 5 μm, v by h
- Sample stage up to 200 kg
Instrument specialty: simultaneous SAXS and WAXS
This branch of the Swedish Materials Science beamline offers world leading instrumentation in simultaneous SAXS and WAXS measurements. The beamline's staff has researched beam stop design. Their research has resulted in a novel beam stop design that is now installed. This novel beam stop blocks the non-scattered beam, without blocking the closely adjacent scattered radiation, and also avoides contributing to nose. Background noise is avoided by a flight tube, which is evacuated. The flight tube is 15 m long to enable an accurate measure of the small angles of scattered signals.
More details will be presented in a CeXS Technical Report that is currently being prepared. The report will present a streamlined method using a furnace sample environment - from planning at the home institute through to results. (Read more here.)
Research case: Catalytic CO oxidation
Catalytic CO oxidation is a seemingly simple reaction between CO and O2 molecules, and is one of the key reactions required of automotive catalytic converters. However, despite decades of investigations, the phase in the catalyst that is responsible for the catalytic activity is still under debate.
Gustafson et al (2018) resolved that debate as part of a relatively straightforward but conclusive study of single crystal Rh and Pd model catalysts. That study made use of simultaneous SAXS and WAXS measurements.
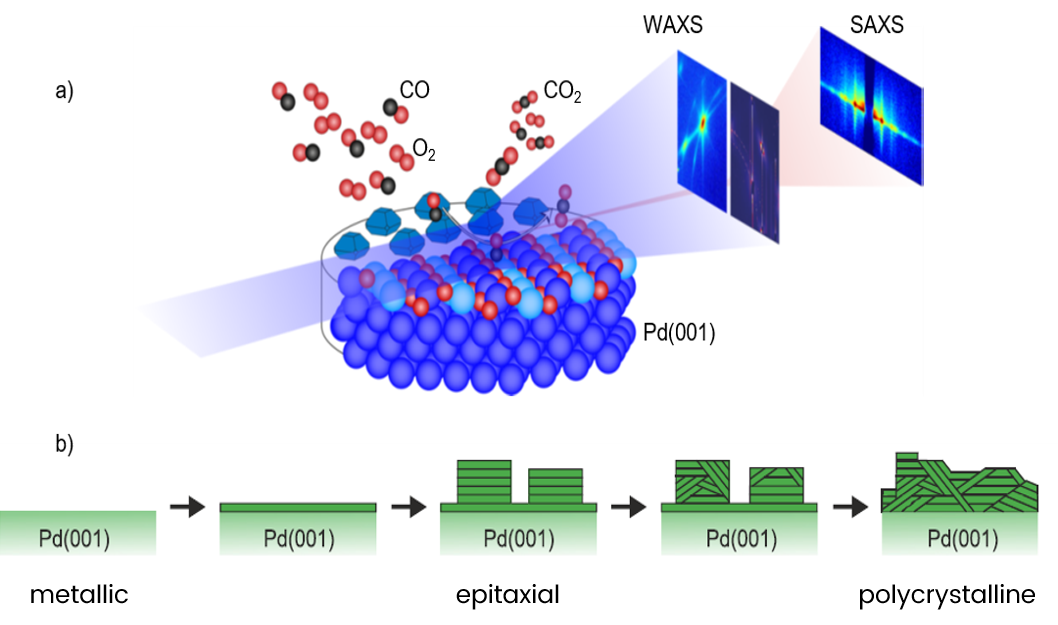
Under in operando conditions, WAXS measurements enabled the identification of the reactions arising from the catalysis process whereas SAXS measurements enabled the identification of the metallic phase of the Rh and Pd actual catalysts.
Gustafson et al (2018) found that for Rh, the oxygen-covered metallic surface is more active than the oxide. For Pd, thin oxide films are at least as active as the metallic surface, but a thicker oxide is less active.
In addition to resolving the long-standing debate, their research results pinpointed important design principles for oxidation catalysts that can prevent catalytic extinction at high oxygen exposures.
Read more about this case in Gustafson, et al. ACS Catal. 8, 4438 (2018).
Further information
For more technical information about the P21.2 branch see this DESY site.



