The P21.1 branch
The P21.1 branch was designed specifically for research in the fields of materials physics and chemistry

The Swedish Materials Science beamline's P21.1 branch was designed specifically for research in the fields of materials physics and chemistry.
ID card
The ID card of this branch is:
- Energy settings: 54, 87, 103 keV
- Photon flux on sample: 2 x 1011 photons/s at 100 keV
- Energy bandwidth: 0.1 %
- Beam size: 1 x 1 mm2
- X-ray diffraction, total scattering
- Sample type: solid, liquid (single crystal, powder, amorphous)
Instrument specialty: Pair distribution function (PDF)
A noteworthy area of expertise of the beamline staff is the total scattering technique of Pair Distribution Function (PDF) analysis. Using PDF it is possible to characterise amorphous materials, particularly the distribution of distances between pairs of particles contained within a given volume.
Research example: Formation and growth of nano particle catalysts
An example of using PDF in research experiments is to monitor the reaction mechanisms of high entry alloy nano particles.
Broge et al (2020) consider high-entropy alloy (HEA) nanoparticles to be particularly promising as tunable catalysts. Catalysis research is a focal area of the materials science field because of the potential to reduce the energy required in many industrial processes. However, catalysts are difficult to design and produce. Broget et al overcame the challenge of forming nanoparticle HEAs in oxygen-rich environments by synthesising Pt-Ir-Pd-Rh-Ru nanoparticles under benign low-temperature solvothermal conditions.
They used in situ X-ray scattering, with PDF data analysis techniques, and transmission electron microscopy to reveal the solvothermal formation mechanism of Pt-Ir-Pd-Rh-Ru nanoparticles.
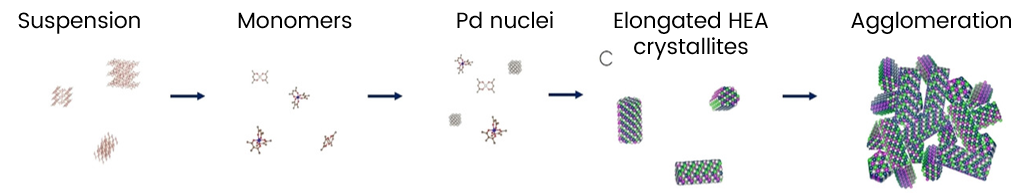
They found that for the individual metal acetylacetonate precursors, the formation of single metal nanoparticles takes place at temperatures spanning from ca. 150 °C for Pd to ca. 350 °C for Ir.
However, for the mixture, homogenous Pt-Ir-Pd-Rh-Ru HEA nanoparticles can be obtained around 200 °C due to autocatalyzed metal reduction at the (111) facets of the forming crystallites. The autocatalytic formation mechanism suggests that many types of HEA nanocatalysts should be accessible with scalable solvothermal reactions, thereby providing broad availability and tunability.
See more information in the article Broge et al. Angew. Chem. Int. Ed. 59, 21920 (2020) .
Further information
For more technical information about the P21.1 branch of the Swedish Materials Science beamline see this DESY site .



