Electrochemical flow cell
The Electrochemical flow cell has been used at both PETRA III and ESRF to follow the evolution of the aluminum oxide film thickness over time, and to study the anodic corrosion behavior of a single-crystalline film of IrO2.
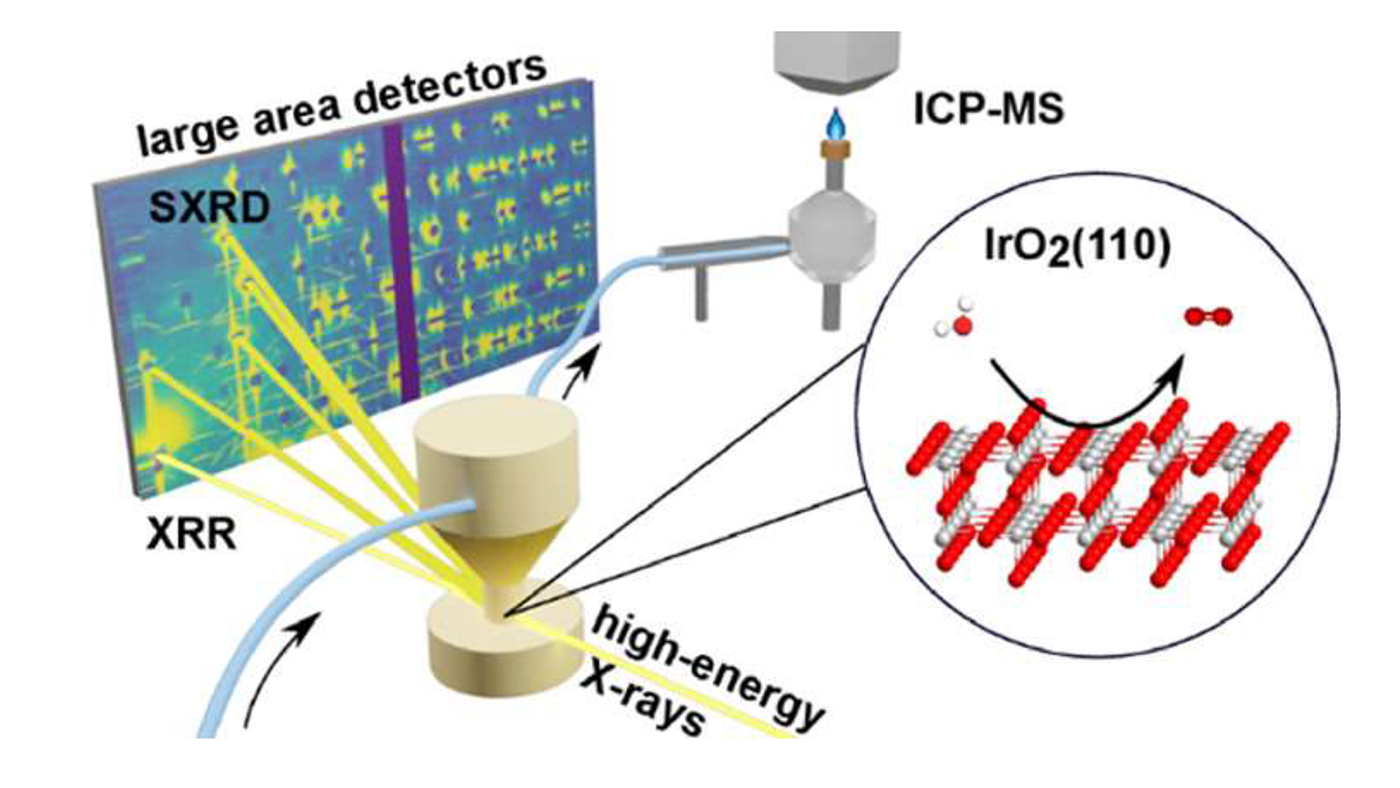
Research examples
Zhang et al. studied the anodization of aluminum alloys (AA 6082 and AA 7075). Using this electrochemical cell, they performed in-situ X-Ray Reflectivity (XRR) during anodization at different applied potential (from open circuit potential to 3.7 V) to follow the evolution of the aluminum oxide film thickness over time. Qualitative trends of the roughness evolution within the aluminum oxide film thickness was also extracted. Experiments were performed at the ID03 beamline at ESRF.
Weber et al. studied the anodic corrosion behavior of a single-crystalline film of IrO2 supported by a slightly bulk-reduced TiO2(110) single crystals by acidic water splitting. They performed operando XRR and Surface X-Ray Diffraction (SXRD) during the corrosion reaction. The combination of these techniques allowed to follow the film stability, by following its thickness and structural evolution. Experiments were performed at the PETRA III Swedish Materials Science beamline, P21.2 branch.
Further information
Read more about this research in the articles:
- F. Zhang, J. Evertsson, F. Bertram, L. Rullik, F. Carla, M. Långberg, E. Lundgren, J. Pan, Integration of electrochemical and synchrotron-based X-ray techniques for in-situ investigation of aluminum anodization, Electrochimica Acta. 241 (2017) 299–308. https://doi.org/10.1016/j.electacta.2017.04.154.
- T. Weber, V. Vonk, D. Escalera-López, G. Abbondanza, A. Larsson, V. Koller, M.J.S. Abb, Z. Hegedüs, T. Bäcker, U. Lienert, G.S. Harlow, A. Stierle, S. Cherevko, E. Lundgren, H. Over, Operando Stability Studies of Ultrathin Single-Crystalline IrO2(110) Films under Acidic Oxygen Evolution Reaction Conditions, ACS Catal. 11 (2021) 12651–12660. https://doi.org/10.1021/acscatal.1c03599.
See the sample environment's design and specifications in
- M. L. Foresti et al. (2006) Electrochim. Acta, Vol. 51, p.5532−5539.



