Device improves stem cell generation and chances for personalized cell therapy
Researchers at KTH Royal Institute of Technology say they have improved on a technique for converting regular skin cells into neural stem cells—an advance which they say helps close the gap for accessible personalized cell-based therapies for Alzheimer's and Parkinson's.
Using a specially-designed microfluidic device, the research team have developed an unprecedented and speedier approach to reprogramming human skin cells into induced pluripotent stem cells (iPSCs), and further transforming them into neural stem cells.
The study’s first author Saumey Jaim says the platform could improve – and lower the cost of – cell therapy, making cells easier to match and be accepted by a patient’s body. The research was reported in Advanced Science by researchers from KTH Royal Institute of Technology.
Anna Herland, the senior author of the study, says the study demonstrated the first-ever case of microfluidics being used to redirect iPSCs toward becoming neural stem cells.
Engineering the transformation from regular cells into neural stem cells is in effect a two-stage process. Using a process that involves exposing cells to biochemical cues, the cells are induced into pluripotent stem cells, which have the power to generate different cell types.
Then they are transferred to a culture medium that mimics the signaling cues and developmental processes involved in formation of the nervous system. This stage, called neural differentiation, redirects cells to commit to being neural stem cells.
The medium for this kind of lab work has been shifting from well plates to microfluidic devices for nearly a decade. Herland says the new platform represents an improvement of microfluidics for both stages, iPSC generation and neural stem cell differentiation.
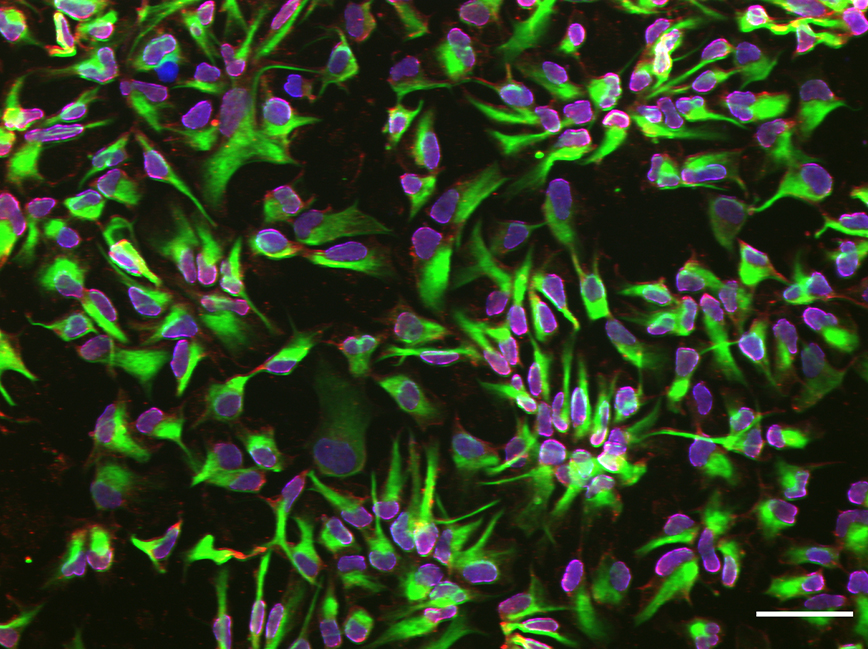
Using cells from a human skin biopsy, they found that the microfluidic platform enabled a boosted commitment to their neural fate at an earlier point than those differentiated in a conventional well plate format.
“We documented that the confined environment of a microfluidic platform boosts neural stem cell generation commitment,” Herland says.
Jain says the microfluidic chip is easy to fabricate using polydimethylsiloxane (PDMS), and its microscale size offers substantial cost savings in terms of reagents and cellular input.
The platform can be easily modified to enable adaptability for differentiation into other cell types, he says. It can be automated, providing a closed system that ensures consistency and reliability in producing highly homogenous cell populations.
“This marks a step towards making personalized cell-based therapies for Alzheimer's and Parkinson's accessible.”
Contributing to the study were researchers from Karolinska Institutet and Lund University collaborating in the VINNOVA-funded consortium IndiCell.
David Callahan callahan[at]kth[dot]se
