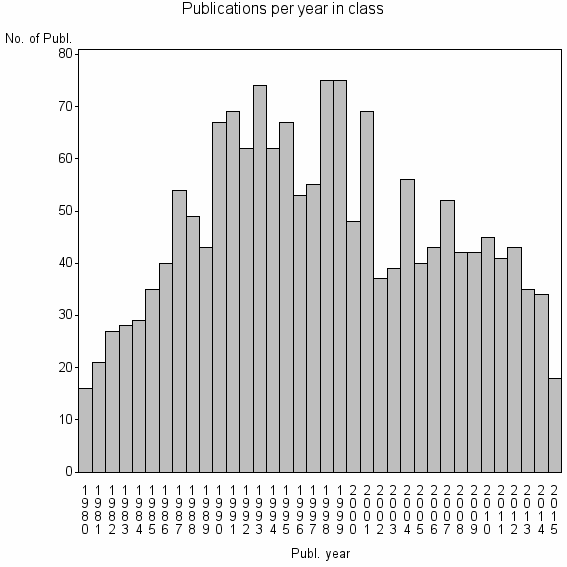
Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
| 4596 | 1685 | 29.5 | 63% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 1009 | 9870 | PERTUSSIS//BORDETELLA PERTUSSIS//WHOOPING COUGH |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | HAEMOPHILUS INFLUENZAE TYPE B | Author keyword | 171 | 59% | 11% | 190 |
| 2 | HIB | Author keyword | 33 | 56% | 2% | 40 |
| 3 | HAEMOPHILUS INFLUENZAE TYPE B VACCINE | Author keyword | 26 | 63% | 2% | 26 |
| 4 | WHOLE CELL PERTUSSIS | Author keyword | 18 | 89% | 0% | 8 |
| 5 | PENTAVALENT VACCINE | Author keyword | 17 | 72% | 1% | 13 |
| 6 | COMBINATION VACCINES | Author keyword | 16 | 43% | 2% | 29 |
| 7 | COMBINATION VACCINE | Author keyword | 15 | 42% | 2% | 28 |
| 8 | HAEMOPHILUS INFLUENZAE B | Author keyword | 14 | 59% | 1% | 16 |
| 9 | HAEMOPHILUS INFLUENZAE | Author keyword | 14 | 11% | 7% | 119 |
| 10 | HAEMOPHILUS INFLUENZAE TYPE B CONJUGATE VACCINE | Author keyword | 14 | 65% | 1% | 13 |
Web of Science journal categories |
Author Key Words |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | PRP T | 109 | 89% | 3% | 49 |
| 2 | HIB | 63 | 76% | 3% | 44 |
| 3 | ACELLULAR PERTUSSIS | 54 | 42% | 6% | 98 |
| 4 | OROPHARYNGEAL CARRIAGE | 47 | 66% | 3% | 43 |
| 5 | COMBINATION VACCINES | 45 | 55% | 3% | 56 |
| 6 | HIB DISEASE | 37 | 75% | 2% | 27 |
| 7 | HBOC VACCINE | 36 | 79% | 1% | 23 |
| 8 | CHILEAN INFANTS | 34 | 93% | 1% | 13 |
| 9 | PROTEIN CONJUGATE VACCINE | 33 | 45% | 3% | 54 |
| 10 | ALASKA NATIVE INFANTS | 32 | 78% | 1% | 21 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references | % act. ref. to same field |
|---|---|---|---|---|
| Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: Global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates | 2000 | 323 | 120 | 79% |
| Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis | 1999 | 132 | 34 | 100% |
| Hoemophilus influenzae type b conjugate vaccine use and effectiveness | 2008 | 63 | 57 | 95% |
| Haemophilus influenzae type b conjugate vaccines | 2004 | 96 | 86 | 79% |
| The UK Hib vaccine experience | 2002 | 87 | 28 | 86% |
| Dose-specific efficacy of Haemophilus influenzae type b conjugate vaccines: a systematic review and meta-analysis of controlled clinical trials | 2012 | 9 | 36 | 81% |
| Carrier priming or suppression: Understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines | 2014 | 7 | 56 | 55% |
| Review of diphtheria, tetanus and pertussis vaccines in clinical development | 2011 | 12 | 11 | 64% |
| Use of vaccines as probes to define disease burden | 2014 | 12 | 45 | 24% |
| Combination vaccines containing DTPa-Hib: impact of IPV and coadministration of CRM 197 conjugates | 2008 | 26 | 101 | 77% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | HAEMOPHILUS REFERENCE UNIT | 11 | 60% | 0.7% | 12 |
| 2 | HIB INITIAT | 4 | 75% | 0.2% | 3 |
| 3 | OXFORD VACCINE GRP | 4 | 14% | 1.4% | 24 |
| 4 | ASSOC AIDE MED PREVENT | 3 | 50% | 0.2% | 4 |
| 5 | CHILDRENS VACCINE INITIAT | 3 | 60% | 0.2% | 3 |
| 6 | 10 S PINE ST | 2 | 67% | 0.1% | 2 |
| 7 | CVD MALI | 2 | 67% | 0.1% | 2 |
| 8 | DEV VACCINS MALI | 2 | 67% | 0.1% | 2 |
| 9 | HAEMOPHILUS REFERENCE | 2 | 67% | 0.1% | 2 |
| 10 | HLTH SERVTSHWANE | 2 | 67% | 0.1% | 2 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000202885 | ICES UOTTAWA//VACCINATION AGE//TUBERCULIN RESPONSE |
| 2 | 0.0000170825 | HAEMOPHILUS INFLUENZAE//NONTYPEABLE HAEMOPHILUS INFLUENZAE//NTHI |
| 3 | 0.0000123551 | PNEUMOCOCCAL POLYSACCHARIDE VACCINE//PNEUMOCOCCAL VACCINE//23 VALENT PNEUMOCOCCAL POLYSACCHARIDE VACCINE |
| 4 | 0.0000104863 | NATIONAL IMMUNIZATION TECHNICAL ADVISORY GROUP NITAG//IMMUNIZATION FINANCING//PROVAC INITIAT |
| 5 | 0.0000091876 | PERTUSSIS//BORDETELLA PERTUSSIS//WHOOPING COUGH |
| 6 | 0.0000090740 | TL 2 RESPONSE//ADCC REACTION//ANTI IG DEXTRAN |
| 7 | 0.0000089140 | BACTERIAL MENINGITIS//MENINGITIS//MICROBIOL EXPT |
| 8 | 0.0000084522 | NEISSERIA MENINGITIDIS//MENINGOCOCCAL DISEASE//MENINGOCOCCAL REFERENCE UNIT |
| 9 | 0.0000083290 | ANTIGENURIA//ANTIGEN DETECTION TESTS//CAPSULAR POLYSACCHARIDE ANTIGENS |
| 10 | 0.0000071467 | IGG SUBCLASSES//MONOGRAPHS IN ALLERGY//IGG SUBCLASS |