Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
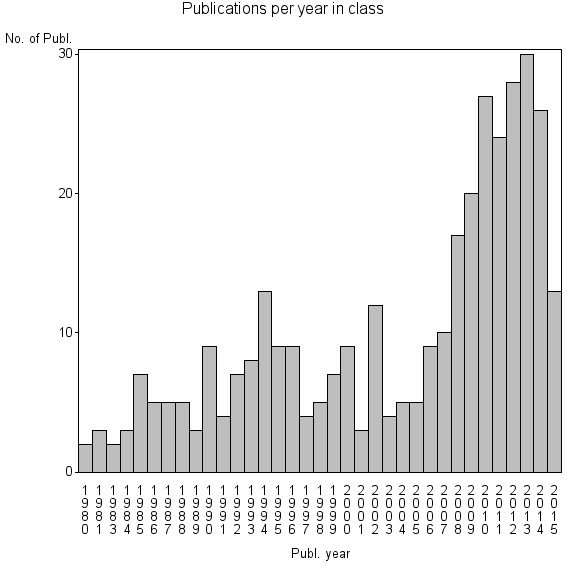
| 21686 | 352 | 33.0 | 55% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 3280 | 1415 | IMMUNOTOXICITY//DEVELOPMENTAL IMMUNOTOXICITY//TGN1412 |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | TGN1412 | Author keyword | 13 | 61% | 4% | 14 |
| 2 | SAFETY PHARMACOLOGY | Author keyword | 6 | 19% | 8% | 28 |
| 3 | REPEAT DOSE TOXICITY STUDIES | Author keyword | 4 | 75% | 1% | 3 |
| 4 | NONCLINICAL TESTING | Author keyword | 3 | 100% | 1% | 3 |
| 5 | CYTOKINE RELEASE ASSAYS | Author keyword | 2 | 67% | 1% | 2 |
| 6 | DCTDA | Address | 2 | 67% | 1% | 2 |
| 7 | NON CLINICAL SAFETY | Author keyword | 2 | 50% | 1% | 3 |
| 8 | 12 MONTH | Author keyword | 1 | 100% | 1% | 2 |
| 9 | CHRONIC TOXICITY STUDIES | Author keyword | 1 | 100% | 1% | 2 |
| 10 | ICRRU | Address | 1 | 50% | 1% | 2 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | TGN1412 | 13 | 61% | 4% | 14 | Search TGN1412 | Search TGN1412 |
| 2 | SAFETY PHARMACOLOGY | 6 | 19% | 8% | 28 | Search SAFETY+PHARMACOLOGY | Search SAFETY+PHARMACOLOGY |
| 3 | REPEAT DOSE TOXICITY STUDIES | 4 | 75% | 1% | 3 | Search REPEAT+DOSE+TOXICITY+STUDIES | Search REPEAT+DOSE+TOXICITY+STUDIES |
| 4 | NONCLINICAL TESTING | 3 | 100% | 1% | 3 | Search NONCLINICAL+TESTING | Search NONCLINICAL+TESTING |
| 5 | CYTOKINE RELEASE ASSAYS | 2 | 67% | 1% | 2 | Search CYTOKINE+RELEASE+ASSAYS | Search CYTOKINE+RELEASE+ASSAYS |
| 6 | NON CLINICAL SAFETY | 2 | 50% | 1% | 3 | Search NON+CLINICAL+SAFETY | Search NON+CLINICAL+SAFETY |
| 7 | 12 MONTH | 1 | 100% | 1% | 2 | Search 12+MONTH | Search 12+MONTH |
| 8 | CHRONIC TOXICITY STUDIES | 1 | 100% | 1% | 2 | Search CHRONIC+TOXICITY+STUDIES | Search CHRONIC+TOXICITY+STUDIES |
| 9 | IMMOBILISED ANTIBODY | 1 | 100% | 1% | 2 | Search IMMOBILISED+ANTIBODY | Search IMMOBILISED+ANTIBODY |
| 10 | IMPLANTED TELEMETRY | 1 | 100% | 1% | 2 | Search IMPLANTED+TELEMETRY | Search IMPLANTED+TELEMETRY |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | BIOTECHNOLOGY DERIVED PHARMACEUTICALS | 12 | 86% | 2% | 6 |
| 2 | MONOCLONAL ANTIBODY TGN1412 | 8 | 33% | 5% | 19 |
| 3 | CHRONIC ANIMAL TOXICITY | 6 | 80% | 1% | 4 |
| 4 | NON RODENT TOXICITY | 6 | 100% | 1% | 4 |
| 5 | TGN1412 | 4 | 28% | 3% | 11 |
| 6 | CD4 TRANSGENIC MICE | 3 | 60% | 1% | 3 |
| 7 | CD28 SUPERAGONISTS | 3 | 35% | 2% | 6 |
| 8 | CYTOKINE STORM | 2 | 16% | 4% | 14 |
| 9 | RELEASE SYNDROME | 2 | 44% | 1% | 4 |
| 10 | 1ST IN HUMAN CLINICAL TRIALS | 1 | 100% | 1% | 2 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| Cytokine release assays: Current practices and future directions | 2014 | 5 | 23 | 65% |
| Relevance of the 1-year dog study in assessing human health risks for registration of pesticides. An update to include pesticides registered in Japan | 2014 | 1 | 6 | 100% |
| Cardiovascular pressure measurement in safety assessment studies: Technology requirements and potential errors | 2014 | 1 | 5 | 80% |
| A retrospective analysis of toxicity studies in dogs and impact on the chronic reference dose for conventional pesticide chemicals | 2010 | 10 | 8 | 100% |
| A 1-year toxicity study in dogs is no longer a scientifically justifiable core data requirement for the safety assessment of pesticides | 2010 | 9 | 8 | 88% |
| Antibody therapeutics - the evolving patent landscape | 2011 | 2 | 1 | 100% |
| The future of non-human primate use in mAb development | 2010 | 12 | 18 | 67% |
| Thirty years of preclinical safety evaluation of biopharmaceuticals: did scientific progress lead to appropriate regulatory guidance? | 2012 | 5 | 13 | 54% |
| Safety of biologics, lessons learnt from TGN1412 | 2009 | 20 | 23 | 39% |
| The enhanced pre- and postnatal study for nonhuman primates: Update and perspectives | 2011 | 9 | 24 | 46% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | DCTDA | 2 | 67% | 0.6% | 2 |
| 2 | ICRRU | 1 | 50% | 0.6% | 2 |
| 3 | CBSS | 1 | 50% | 0.3% | 1 |
| 4 | CORP QUAL CONTROL | 1 | 50% | 0.3% | 1 |
| 5 | FED HLTH PROTECT CONSUMERS VET MED BGVV | 1 | 50% | 0.3% | 1 |
| 6 | NONCLIN EVALUAT EXPERT COMM | 1 | 50% | 0.3% | 1 |
| 7 | QTEST S LLC | 1 | 50% | 0.3% | 1 |
| 8 | SECT PHARMACOL TOXICOL BIOTECHNOL FTBB | 1 | 50% | 0.3% | 1 |
| 9 | TRANSLAT SCI SAFETY | 1 | 50% | 0.3% | 1 |
| 10 | EXCELLENCE CARDIOVASC SAFETY MECHANIST | 1 | 29% | 0.6% | 2 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000134309 | DEVELOPMENTAL IMMUNOTOXICITY//JUVENILE ANIMAL STUDIES//IMMUNOTOXICITY GUIDELINES |
| 2 | 0.0000118188 | BIOSIMILARS//BIOSIMILAR//ANTI ERYTHROPOIETIN ANTIBODIES |
| 3 | 0.0000099770 | FCRN//NEONATAL FC RECEPTOR//TARGET MEDIATED DRUG DISPOSITION |
| 4 | 0.0000099718 | COMPUTAT TOXICOL//ALTERNAT ANIM TESTING//ALTEX-ALTERNATIVES TO ANIMAL EXPERIMENTATION |
| 5 | 0.0000081597 | CLINICAL TRIAL RISK//CONTINUOUS IMPROVEMENT//STRATEGIE IND |
| 6 | 0.0000075808 | THREE RS//SYRCLE//DIRECTIVE 86 609 EEC |
| 7 | 0.0000071962 | TORSADES DE POINTES//TORSADE DE POINTES//ELECT ENGN CYBERNET |
| 8 | 0.0000062368 | HYBRIDIZATION TECHNOLOGY//IMMUNOLIGAND ASSAY//BIOASSAY GRP |
| 9 | 0.0000060371 | 2 3 4 DIARYL 5 OXO 1 2 4 TRIAZINE 6 YLIDENEACETATES//3 4 DIARYL 1 2 4 TRIAZOLO 5 CARBOXYLATES//ADEUOVIRUS |
| 10 | 0.0000057234 | DRUG LAG//OFF PHARMACEUT IND//JAPANESE CLINICAL TRIALS |