Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
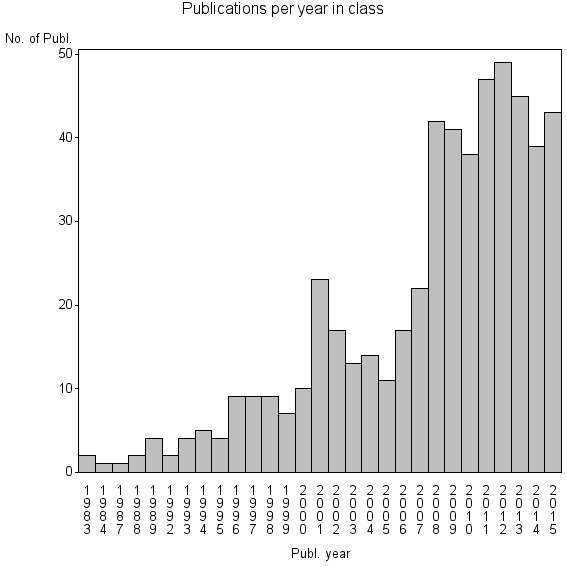
| 17553 | 530 | 29.0 | 72% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 970 | 10197 | CAUSAL INFERENCE//PRINCIPAL STRATIFICATION//STATISTICS IN MEDICINE |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | SURROGATE ENDPOINT | Author keyword | 21 | 36% | 9% | 47 |
| 2 | PRENTICE CRITERION | Author keyword | 14 | 100% | 1% | 7 |
| 3 | PRENTICE CRITERIA | Author keyword | 12 | 86% | 1% | 6 |
| 4 | SURROGATE ENDPOINTS | Author keyword | 10 | 29% | 5% | 28 |
| 5 | LIKELIHOOD REDUCTION FACTOR | Author keyword | 9 | 83% | 1% | 5 |
| 6 | POST PROGRESSION SURVIVAL | Author keyword | 9 | 67% | 2% | 8 |
| 7 | META ANALYTIC APPROACH | Author keyword | 6 | 80% | 1% | 4 |
| 8 | BIOMETR 5 | Address | 5 | 47% | 2% | 8 |
| 9 | SURROGATE OUTCOME | Author keyword | 4 | 42% | 2% | 8 |
| 10 | ENDPOINT ADJUDICATION | Author keyword | 4 | 75% | 1% | 3 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | SURROGATE ENDPOINT | 21 | 36% | 9% | 47 | Search SURROGATE+ENDPOINT | Search SURROGATE+ENDPOINT |
| 2 | PRENTICE CRITERION | 14 | 100% | 1% | 7 | Search PRENTICE+CRITERION | Search PRENTICE+CRITERION |
| 3 | PRENTICE CRITERIA | 12 | 86% | 1% | 6 | Search PRENTICE+CRITERIA | Search PRENTICE+CRITERIA |
| 4 | SURROGATE ENDPOINTS | 10 | 29% | 5% | 28 | Search SURROGATE+ENDPOINTS | Search SURROGATE+ENDPOINTS |
| 5 | LIKELIHOOD REDUCTION FACTOR | 9 | 83% | 1% | 5 | Search LIKELIHOOD+REDUCTION+FACTOR | Search LIKELIHOOD+REDUCTION+FACTOR |
| 6 | POST PROGRESSION SURVIVAL | 9 | 67% | 2% | 8 | Search POST+PROGRESSION+SURVIVAL | Search POST+PROGRESSION+SURVIVAL |
| 7 | META ANALYTIC APPROACH | 6 | 80% | 1% | 4 | Search META+ANALYTIC+APPROACH | Search META+ANALYTIC+APPROACH |
| 8 | SURROGATE OUTCOME | 4 | 42% | 2% | 8 | Search SURROGATE+OUTCOME | Search SURROGATE+OUTCOME |
| 9 | ENDPOINT ADJUDICATION | 4 | 75% | 1% | 3 | Search ENDPOINT+ADJUDICATION | Search ENDPOINT+ADJUDICATION |
| 10 | TRUE ENDPOINT | 4 | 75% | 1% | 3 | Search TRUE+ENDPOINT | Search TRUE+ENDPOINT |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | EVENTS COMMITTEE | 7 | 64% | 1% | 7 |
| 2 | CLINICAL EVENTS COMMITTEE | 7 | 67% | 1% | 6 |
| 3 | INFARCTION END POINTS | 6 | 50% | 2% | 8 |
| 4 | ENDPOINTS | 5 | 24% | 4% | 19 |
| 5 | 18 RANDOMIZED TRIALS | 4 | 30% | 2% | 10 |
| 6 | MARKER EVALUATION | 3 | 100% | 1% | 3 |
| 7 | MISMEASURED OUTCOMES | 3 | 100% | 1% | 3 |
| 8 | HIERARCHICAL LEVELS | 3 | 50% | 1% | 4 |
| 9 | OBJECTIVE RESPONSE | 3 | 50% | 1% | 4 |
| 10 | ADJUVANT COLON CANCER | 2 | 67% | 0% | 2 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| Accelerated Approval of Oncology Products: The Food and Drug Administration Experience | 2011 | 50 | 3 | 67% |
| A systematic review evaluating the relationship between progression free survival and post progression survival in advanced ovarian cancer | 2012 | 11 | 14 | 86% |
| Review of meta-analyses evaluating surrogate endpoints for overall survival in oncology | 2012 | 14 | 48 | 60% |
| Outcomes and endpoints in trials of cancer treatment: the past, present, and future | 2015 | 4 | 95 | 22% |
| Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature | 2010 | 59 | 40 | 40% |
| Biomarkers and surrogate end points-the challenge of statistical validation | 2010 | 80 | 56 | 30% |
| Overall Survival and Post-Progression Survival in Advanced Breast Cancer: A Review of Recent Randomized Clinical Trials | 2010 | 52 | 26 | 38% |
| The Strength of Association Between Surrogate End Points and Survival in Oncology A Systematic Review of Trial-Level Meta-analyses | 2015 | 1 | 78 | 53% |
| Evaluation of Blinded Independent Central Review of Tumor Progression in Oncology Clinical Trials: A Meta-analysis | 2013 | 3 | 10 | 100% |
| Statistical evaluation of biomarkers as surrogate endpoints: a literature review | 2006 | 69 | 58 | 53% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | BIOMETR 5 | 5 | 47% | 1.5% | 8 |
| 2 | ONCOL THER Y AREA | 4 | 75% | 0.6% | 3 |
| 3 | DATA CANC CLIN TRIALS | 3 | 60% | 0.6% | 3 |
| 4 | BIOSTAT QUAL LIFE UNIT EA4184 | 1 | 100% | 0.4% | 2 |
| 5 | METHODOL QUAL LIFE UNIT ONCOL EA3181 | 1 | 100% | 0.4% | 2 |
| 6 | BIOSTAT E0338 | 1 | 50% | 0.2% | 1 |
| 7 | BRANCH BIOMETR | 1 | 50% | 0.2% | 1 |
| 8 | CDER OTS OB DBV | 1 | 50% | 0.2% | 1 |
| 9 | DECIS SUPPORT UNIT | 1 | 50% | 0.2% | 1 |
| 10 | DIGEST ONCOL GASTROENTEROL | 1 | 50% | 0.2% | 1 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000218969 | RECIST//RECIST 11//TUMOR MEASUREMENT |
| 2 | 0.0000138712 | INTERACTION CONDITION//TARGETED CLINICAL TRIALS//INFLUENCE CONDITION |
| 3 | 0.0000119299 | CAUSAL INFERENCE//PRINCIPAL STRATIFICATION//TARGETED MAXIMUM LIKELIHOOD ESTIMATION |
| 4 | 0.0000098348 | TSU 68//APATINIB//MUCOCUTANEOUS TOXICITIES |
| 5 | 0.0000081925 | WORST RANK SCORES//REPRODUCIBILITY PROBABILITY//BOUNDED OUTCOME SCORES |
| 6 | 0.0000071575 | ADAPTIVE TREATMENT STRATEGIES//DYNAMIC TREATMENT REGIME//DYNAMIC TREATMENT REGIMES |
| 7 | 0.0000069487 | RESPONSE SELECTIVE SAMPLING//CASE COHORT DESIGN//OUTCOME DEPENDENT SAMPLING |
| 8 | 0.0000068503 | CONDITIONAL POWER//INTERIM ANALYSIS//SAMPLE SIZE RE ESTIMATION |
| 9 | 0.0000063325 | INTERVAL CENSORING//CURRENT STATUS DATA//INTERVAL CENSORED DATA |
| 10 | 0.0000060736 | DRUG LAG//OFF PHARMACEUT IND//JAPANESE CLINICAL TRIALS |